
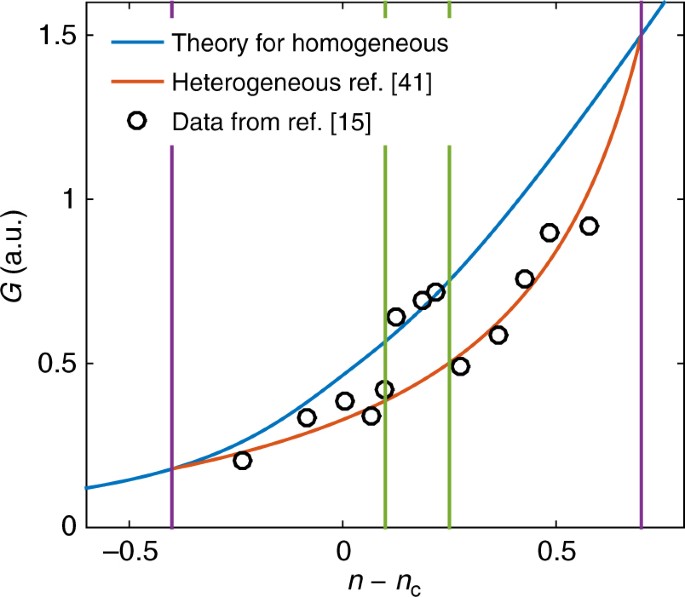
The essence of the present controversy may be highlighted by the following questions: Does a glass possess a finite residual entropy at absolute zero? And how does the entropy of a glass forming system change, abruptly, at the glass transition? Based on the computation of energy landscapes and the concept of broken ergodicity, the glassy state is described in terms of a liquid confined to a single metabasin of the configurational phase space with an entropy loss – but no latent heat – accompanying the glass transition. The role of entropy in undercooled and frozen-in liquids has since been the subject of many debates, with researchers arguing from the viewpoints of thermodynamics, statistical mechanics, and kinetics. Approximately 20 years later, Kauzmann published his paper on the nature of the glassy state and the behavior of liquids at low temperatures, from which the scientific world deduced the “Kauzmann Paradox”, wherein the extrapolated entropy of an undercooled liquid apparently approaches a value below the one of the isochemical crystalline state. ) and others – led to a reformulation of the 3rd law by Simon in 1927. The debate focused on the concept of internal equilibrium and – based on pioneering experimental work by Simon on glassy glycerol and silica, Giauque and coworkers (e.g. Since, scientists have been wrestling with the possibility of finite entropy – more precisely: of a finite positive entropy difference to the universal limit – at absolute zero temperature for certain types of materials. The statement is most conveniently accounted for by defining this universal limit as zero. The eventual formulation of the third law, however, goes back to Planck, who in 1910 interpreted Nernst’s theorem: “Upon infinitely decreasing temperature, the entropy of any pure condensed substance approaches a limit which is independent of pressure, aggregate state, and chemical modification” (translated from German the term “chemical modification” is used as a synonym of “chemical composition”). In 1921 the Nobel Prize in Chemistry 1920 was bestowed upon him “in recognition of his work in thermochemistry. The basis of today’s controversy is Nernst’s theorem which he presented in 1905, one day before Christmas, at a meeting of the Königliche Gesellschaft der Wissenschaften (Göttingen, Germany, ) and a year later, on December 20, 1906, at a meeting of the Königlich Preussische Akademie der Wissenschaften (Berlin, Germany, ).
This first workshop was endorsed by the newly founded Technical Committee 08 of the International Comission on Glass “Relaxation Phenomena in Glasses” and supported locally by the Slovak Glass Society. Lothar Wondraczek and Reinhard Conradt, both from Germany, agreed to organize and chair it. As a consequence, representatives from Corning’s European Technology Center, Avon, France spontaneously opted to sponsor the first international workshop on glass and entropy, and Profs.
Entropy synonym series#
Since then, none of the controversial issues could be resolved in a satisfactory way, and more and more researchers from different fields (glass and polymer sciences, statistical mechanics, computational simulation) agreed that there is a need to meet, on a regular and long-term basis, within the frame of a series of workshops specifically dedicated to glass and its entropy. There, controversial viewpoints and opinions on the entropy of the glassy state surfaced in a highly emotional scientific debate.

The idea to organize an international workshop dedicated to glass and entropy was born during the session “Thermodynamics, Rheology, and Glass Transition” held at the XXIst International Congress on Glass, Strasbourg 2007.


 0 kommentar(er)
0 kommentar(er)
